What awaits you in this article: Hybrid Zn-Mn(11 wt.%) alloy coatings containing in their matrix stabilized polymeric micelles (SPM) are electrodeposited from a sulfate bath. SPM are added to the electrolytic bath in concentrations of 0.1 or 0.3 wt.%, respectively. The coatings are additionally treated in Cr(III)-based solutions for obtaining of surface conversion layers (CL) aiming at receiving of better corrosion resistance. Surface morphology of the hybrid coatings (with or without additional conversion layer) is presented by SEM. The protective characteristics are evaluated with potentiodynamic polarization (PD) and polarization resistance (Rp) measurements in a model corrosion medium of 3% NaCl solution. Experimental results demonstrate the better corrosion resistance and the greater protective ability of the hybrid coatings obtained from electrolyte with 0.1 wt.% SPM, compared to the ordinary Zn-Mn alloy.
1 Introduction
The corrosion resistance of the low-carbon and low-alloyed steels is of great importance for the civil engineering, industry, household etc. [1–3]. In order to ensure long service life of the equipment the steel is often protected by application of different coatings – for example galvanic, organic, hybrid, conversion ones etc. [4–12]. Zinc-manganese alloy coatings characterize with high corrosion resistance and protective ability also in cases when local corrosion processes appear [13–15]. Generally, these coatings are electrochemically obtained mainly from sulfate or chloride based electrolytic baths [16]. Due to their good protective characteristics they find wide practical application in different industrial areas which require exploitation at heavy conditions.
Contrary to many other metals used for the electrodeposition of zinc based alloys (like Ni, Co, etc.) Mn characterizes with more negative standard potential compared to the Zn so the potential of the Zn-Mn alloy is less noble than that of the ordinary zinc.
The improvement of the protective ability of Zn-Mn alloy coating can be realized by its additional treatment (immersion) in solutions for chemical passivation, the latter containing for example Cr (III)-compounds [17–21]. As a result a conversion layer (CL) with lower susceptibility against aggressive corrosion media appears which can be further used as a basis for organic paints or lacquers. As well known from some literature sources surface conversion layers significantly decrease the dissolution rate of the galvanic coatings [22–25].
The development of the nanotechnologies in the last 20–25 years led to intensive investigation of innovative electrodeposited materials for example composite ones aiming at improvement of their physical-mechanical and protective properties. The obtaining of these materials is generally based on the incorporation and distribution of inorganic or organic particles in the main matrix (metal or alloy) and appearance of a second phase in it leading to increased hardness, corrosion resistance, wear resistance, etc. One new possibility is to embed stabilized polymeric micelles (SPM) in the metal receiving in such a way the so called hybrid coatings [26–30]. These SPM are “core-shell” type where the core is the hydrophobic part and the shell – the hydrophilic one. SPM used can be based for example on polyethylene oxide (PEO), polypropylene oxide (PPO), polyhydroxy–ethylmetacrylate (PHEMA), polystyrene (PS) etc. They can be additionally stabilized either by core or shell cross-linking by forming of chemical bonds or by applying of physical interactions [31].
In the present study the obtaining of hybrid Zn-Mn (11wt. %) alloy coatings with incorporated SPM is demonstrated and discussed. These coatings are additionally treated in environmentally friendly Cr (III)-based solutions for chemical passivating, i.e. for obtaining of transparent (TCL), green (GCL) or black (BCL) conversion layers, respectively. The corrosion behavior of the hybrid coatings (with or without additional CL) is investigated and estimated in a model corrosion medium with chlorine ions as corrosion activators. For comparison, also ordinary Zn-Mn (11wt. %) alloy coatings are treated in the same conversion solutions and investigated with the same experimental methods.
2 Materials and methods
2.1 Sample preparation
Low-carbon steel plates with dimensions 20 x 10 x 1 mm are used for the electrodeposition of the sample coatings – hybrid or ordinary ones. The substrates are subjected to several preliminary treatments like mechanical polishing with sandpaper, followed by degreasing, washing with tap water, rinsing with distilled water and drying at room temperature.
2.2 Electrolytes and electrodeposition conditions
Electrodeposition process is carried out in a starting electrolyte (SE) and in a double-chamber electrolytic glass cell of 300 ml volume, current density of 2 A/dm2, pH ~ 5 and circulation speed of 150 rpm. The composition of the electrolyte is, g/l:
ZnSO4 • 7H2O – 10,0;
MnSO4 • H2O – 100,0;
(NH4)2SO4 – 60,0
and wetting agent (additive AZ1) – 40 ml/l. The hybrid coatings are obtained from SE with an addition of 1g/l or 3 g/l SPM, respectively. The ordinary alloy and both hybrid coatings obtained have an average thickness of ~11-12 µm and are designated as Zn-Mn, Zn-Mn 0.1 or Zn-Mn 0.3 samples.
Metallurgical zinc plate is used as anode, low-carbon steel plate as a cathode and a platinum plate serves as a counter electrode. Under these conditions the alloy Zn-Mn with 11 wt. % manganese is obtained [14, 16]. This galvanic alloy consists mainly of the intermetallic phase δ1 and of small amounts of zinc phase [14, 16].
2.3 Stabilized polymeric micelles (SPM)
SPM used in the present investigations are obtained from commercially available products like amphiphilic three block co-polymers of the type PEO75PPO30PEO75 [poly (ethylene oxide)-b-poly (propylene oxide)-b-poly (ethylene oxide)]. The latter can form spontaneously core-shell micelles in aqueous media. The hydrophobic core consists of PPO segments while the shell is dominated by hydrated PEO chains. The procedure is described elsewhere [32] and includes consecutive put in of hydrophobic functional monomer (pentaerythritol tetraacrylate – PETA) in the micelles followed by polymerizing via UV-irradiation.
This treatment ensures the appearance of a semi-interpenetrating polymeric network (availability of specific netted zones in the micelle). Finally, SPM are dialyzed against distilled water and added to the electrolytes in both concentrations mentioned above – the electrolytic baths are designated as SE 0.1 and SE 0.3.
SPM obtained via this procedure have a spherical shape and sizes of about 200 nm [31]. Generally, SPM can be classified as electrically neutral (non-charged) but under the influence of applied external electrical source they can move electrophoretically to the cathode according to some literature models (for example “ionic cloud” etc.) presented and described elsewhere [33–36].
2.4 Chemical passivating
Three chemical solutions for obtaining of black, transparent or green Cr (III) based conversion layers are used for the surface treatment of Zn-Mn, Zn-Mn 0.1 and Zn-Mn 0.3 coatings. The color and the adhesion of CL obtained depend strongly on the composition, concentration of the active components and on the immersion time. The solutions contain as a main ingredient a tri-valence chromium salt and differ in their composition as presented below:
- Solution for obtaining of transparent CL (TCL) – c ontains also Co2+-compound
- Solution for obtaining of black CL (BCL) – contains also AgNO3 and H3PO4
- Solution for obtaining of green CL (GCL) – contains also Na2S2O5.
All the solutions applied are additionally treated with HNO3 (50 %) aiming at receiving of a suitable and stable exploitation pH value up to 2,0 for TCL and GCL as well as up to 1,7 for BCL, respectively. Immersion time for all investigated probes is specified to be 30 seconds.
After treatment in the conversion solutions and subtraction the specimens from it the chromated samples are rinsed with distilled water and dried at about 60–65 °C. Chemically passivated coatings are ready for corrosion treatment after 24 h – known as “time for ripen” which results in better physical properties (hardness, hydrophobicity and wear-resistance).
Finally, it can be supposed that in the case of both hybrid coatings the newly obtained CL will cover only the metallic parts of the alloy leaving the distributed SPM as uncovered with chromate layer areas.
2.5 Corrosion medium and reproducibility
A model corrosion medium of free aerated 3 % NaCl solution at ambient temperature is used during the investigations. The results obtained are an average of 5 samples per type, i.e. for each measurement 5 replicates of a Zn-Mn, Zn-Mn 0.1 and Zn-Mn 0.3 coatings (without or with CL) are conditioned.
2.6 Surface morphology
Scanning electron microscope JEOL JSM-6390, Japan is used for the estimation of the surface morphology of the investigated samples.
2.7 Electrochemical characterization
The investigations are carried out in a three-electrode experimental cell with Luggin capillary for minimizing the ohmic resistance of the medium. Corrosion potentials are measured with respect to the saturated calomel electrode (SCE) and platinum plate serves as a counter. Following methods are applied:
2.7.1 Potentiodynamic polarization (PD) curves
Aiming at application of accelerated corrosion tests potentiodynamic polarization investigations are carried out using an apparature VersaStat-4 at polarization step of 1 mV/s. The polarization of the sample (working electrode) starts at a value of -100 mV in negative direction toward the corrosion potential (Ecorr), subsequently passing through Ecorr and further polarizing the sample in the anodic direction.
2.7.2 Polarization resistance (Rp) measurements
The protective ability of the electrodeposits for prolonged period of time is estimated by applying of polarization resistance (Rp) measurements according to Stern-Geary equation [37] using a special constructed device – “Corrovit” unit. Having in mind the meaning of the equation above it follows that higher Rp value corresponds to higher corrosion resistance and to lower corrosion rate (corrosion current density) as well.
3 Results and Discussion
3.1 Scanning electron microscopy (SEM)
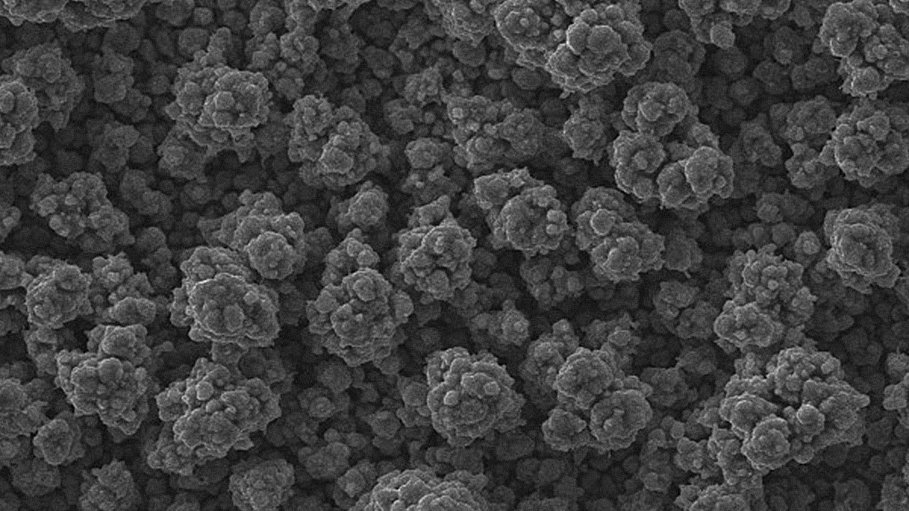
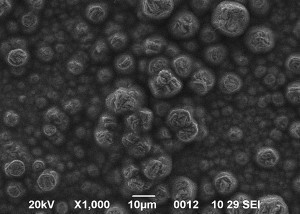
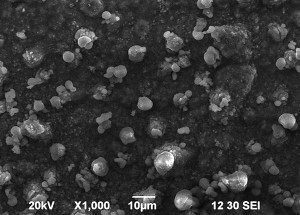
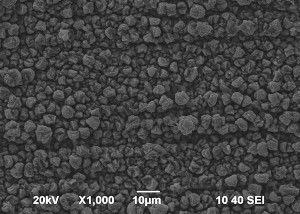
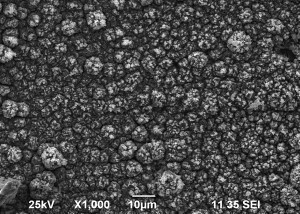
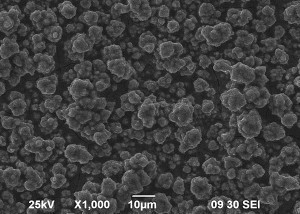
SEM images of Zn-Mn, Zn-Mn 0.1 and Zn-Mn 0.3 are presented in Figure 1. Surface morphology is of great importance for the protective properties of the coatings. The surface of Zn-Mn consists of different in their size aggregates – up to 15 μm. Its surface looks rough and uneven. The addition of 1 g/l SPM to SE changes the morphology leading to smaller grains and greater uniformity. Higher SPM concentration in the bath – 3 g/l – results in more uneven surface morphology and appearing of greater agglomerates accompanied by concaved and convex areas.
The surface morphology of the ordinary and hybrid coatings additionally treated in solutions for chemical passivating is presented in Figures 2–4. Figure 2 demonstrates Zn-Mn, Zn-Mn 0.1 and Zn-Mn 0.3 samples with additional TCL. The surfaces of the galvanic alloy and of Zn-Mn 0.1 coating are relatively close in their view. The chromated Zn-Mn 0.3 hybrid looks more rough and shaggy with several deep concave zones – similar to the case without additional CL (Fig. 1). Compared to Figure 1 the chromated coatings look in general smoother most probably as a result of the low pH value of the conversion solution applied (better expressed “smoothing” action).
The images of Zn-Mn, Zn-Mn 0.1 and Zn-Mn 0.3 coatings after treatment in the chemical solution for obtaining of black CL (BCL) are shown in Figure 3. The surface of Zn-Mn/BCL looks very uneven and is covered with some greater agglomerates with sizes up to 10 μm the latter projecting over the plane of the coating itself. The chromated hybrid coatings Zn-Mn 0.1 and Zn-Mn 0.3 show quite smooth morphology and the surface is more even compared to the galvanic alloy. It seems that part of the agglomerations is dissolved as a result of the lower pH value of this solution (more aggressive action) leading to decreasing in size.
The coating samples treated in the solution for green CL (GCL) are shown in Figure 4. Again, the ordinary alloy coating contains predominantly greater “polished-like” aggregates with spherical shape like in the case of TCL. Chromated Zn-Mn 0.1 and Zn-Mn 0.3 coatings have similar morphology while many of the aggregates are however smaller in size compared to Zn-Mn/GCL. Most probably, these differences in the surface morphology follow mainly from the composition of the applied conversion solution and pH value.
3.2 Potentiodynamic (PD) polarization curves
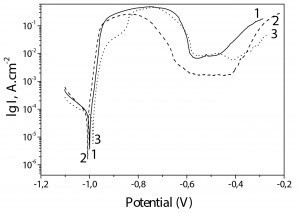
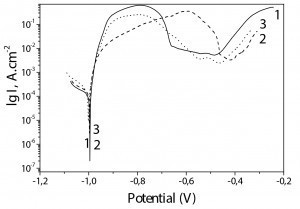
The results obtained via this method for the non-chromated Zn-Mn as well as for Zn-Mn 0.1 and Zn-Mn 0.3 coatings are demonstrated in Figure 5.
As observed, the corrosion potentials for all samples are close. However, the incorporated SPM influence the course of the anodic curves leading to well expressed passive zone for Zn-Mn 0.1 and Zn-Mn 0.3.
In general, the anodic curve of Zn-Mn 0.1 sample (curve 2) characterizes with better parameters – wider passive zone (about 200-230 mV length), earlier passivation (at potential about -0,8 V), and lower anodic current density value in the passive area – 2 • 10-3 A • cm-2.
The chromated ordinary Zn-Mn alloy (curve 1) has the narrowest passive zone and the anodic current density value is comparable with the same parameter of Zn-Mn 0.3 (~.2 • 10–2 A • cm–2) – curve 3. Corrosion current density values (Icorr) of the individual coatings are calculated as follows:
Zn-Mn – 8,3 • 10–5 A • cm–2
Zn-Mn 0.1 - 5,6 • 10–5 A • cm–2
Zn-Mn 0.3 - 5,8 • 10–5 A • cm–2.
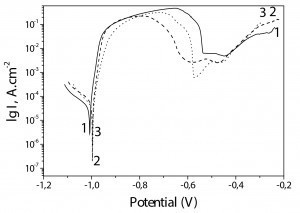
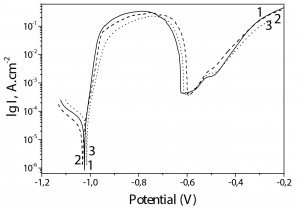
The results obtained for the chromated Zn-Mn, Zn-Mn 0.1 and Zn-Mn 0.3 coatings with TCL are shown in Figure 6.
Once again, the corrosion potentials and corrosion current values of all investigated coatings samples are very close and the main differences can be registered in the anodic area. Generally, the corrosion current values of the three samples are lower compared to the case without CL (Fig. 5):
Zn-Mn – 4,1 • 10–5 A • cm–2
Zn-Mn 0.1 –3,0 • 10–5 A • cm–2
Zn-Mn 0.3 – 3,8 • 10–5 A • cm–2.
The ordinary Zn-Mn alloy (curve 1) distinguishes also here with well expressed passive zone with a length of ~100 mV. Zn-Mn 0.1 coating (curve 2) has a passive zone with a length similar to the case without CL. Contrary to this, Zn-Mn 0.3 (curve 3) does not demonstrate a real passive zone but only decreasing of the anodic current for a short potential interval.
The PD curves of the alloy coating samples treated in solution for black CL are shown in Figure 7. The Zn-Mn (curve 1) and Zn-Mn 0.3 (curve 3) coatings have very close anodic trend in the zone after the corrosion potential. Zn-Mn 0.1 coating (curve 2) distinguishes in the range -1V up to -0,7 V with lower anodic current density value the latter being a sign for slowly dissolution process. This hybrid coating shows also the shortest passive zone in the anodic region. Corrosion current density values (Icorr) of the investigated coatings are as follows:
Zn-Mn – 3,2 • 10–4 A • cm–2
Zn-Mn 0.1 – 1,0 • 10–4 A • cm–2
Zn-Mn 0.3 – 1,6 • 10–4 A • cm–2.
The results for the samples with green CL are shown in Figure 8. In that case all chromated coatings have close anodic behavior having in mind the shape and the electrochemical parameters of the curves. The corrosion current density of Zn-Mn 0.3 (curve 3) seems to be higher compared to both other coatings. Most probably the conversion solution for green passivation transforms the surface more readily and the surface morphology of all the treated samples is very close which is partially confirmed by the SEM investigations.
The corrosion potentials of the coatings in that case are more negative compared to all other types of coatings without or with additional CL. This fact characterizes them as more “sacrificial type” and they can be successfully applied for protection of steel against corrosion. Corrosion current density values (Icorr) are as follows:
Zn-Mn – 4,2 • 10–5 A • cm–2
Zn-Mn 0.1 – 1,9 • 10–5 A • cm–2
Zn-Mn 0.3 – 4,5 • 10–5 A • cm–2.
Following conclusions can be summarized from these results about the corrosion resistance and protective ability of the coatings:
(i) Zn-Mn, Zn-Mn 0.1 and Zn-Mn 0.3 coatings with BCL show the highest Icorr values and increased corrosion rate – the reason is most probably the lowest pH value of the conversion solution (1,7) which lead to accelerated dissolution of the coating during the immersion time and to (probably) thinner thickness; (ii) the best corrosion resistance calculated from the PD curves demonstrate generally the coatings with GCL, followed by these with TCL and the non-chromated ones.
3.3 Polarization resistance (Rp) measurements
Having in mind the abovementioned the Rp measurements are carried out only with selected coating samples the results obtained being presented in Figure 9.
 Fig. 9: Rp measurements for coatings with different CL: 1 – Zn-Mn (TCL); 2 - Zn-Mn 0.1 (TCL); 3 - Zn-Mn (GCL); 4 - Zn-Mn 0.1 (GCL)
Fig. 9: Rp measurements for coatings with different CL: 1 – Zn-Mn (TCL); 2 - Zn-Mn 0.1 (TCL); 3 - Zn-Mn (GCL); 4 - Zn-Mn 0.1 (GCL)
It can be registered that ordinary and hybrid alloy samples with TCL demonstrate similar trend of the polarization resistance changes during the investigated period (compare curves 1 and 2). During the first ~400 h the Rp values of Zn-Mn 0.1/TCL are higher – about 2500 ohm • cm2 – and thereafter this parameter decreases till 1000 ohm • cm2. In comparison, the polarization resistance values of the ordinary chromated alloy are lower during the whole period (curve 1).
Contrary to this, both coating types with GCL distinguish with relatively lower polarization resistance at the beginning of the test but thereafter their trend strongly increase up to about 3500 ohm • cm2 for Zn-Mn/GCL and about 4000 ohm • cm2 for Zn-Mn 0.1/GCL.
The differences observed can be attributed to the influence of SPM on the alloy matrix (mainly changes in the surface morphology) as well as to the quality of the chromating layer covering the surface.
It is well known from different literature sources that the well expressed protective ability of the zinc and its alloys (for example Zn-Mn, Zn-Co etc.) in chloride containing medium is a result of the appearance of relative unifrom and dense film of corrosion products – mainly zinc hydroxide chloride (ZHC) [13–15, 38, 39]. This compound has a low product of solubility value and ensures a barrier effect against the penetration of the chloride ions deeply inside.
When SPM also present in the metal/alloy matrix a mixed protective layer consisting of corrosion products (ZHC) and polymeric particles appear which additionally improves the corrosion resistance and protective ability of the hybrid coating [31]. The combination of this mixed layer and conversion layer areas will significantly decrease the corrosion rate leading to extended service life of the protected details.
4 Conclusions
The presented results show the positive influence of the incorporation of stabilized polymeric micelles in the matrix of Zn-Mn (11 wt. %) alloy. SEM investigations lead to the conclusion that if SPM are added to the electrolytic bath in its lower concentration (1 g/l) a decrease of the crystallite sizes of the alloy can be observed making the surface morphology more even. Coatings obtained from SE 0.3 are more uneven and with greater crystallite sizes demonstrating also worsen electrochemical parameters in comparison to Zn-Mn 0.1 samples.
The ordinary and hybrid alloy coatings allow to be additionally treated with solutions for obtaining of different surface conversion layers the latter leading to better corrosion resistance and protective ability in a model test medium with chlorine ions as corrosion activators. Better results concerning the protective ability against the steel substrate demonstrate the samples with GCL followed by these with TCL. The reason for this seems to be most probably the higher pH value in the case of both these conversion solution – 2; contrary to BCL where this parameter is 1,7. In the latter case more pronounced dissolution process can be expected which will worsen the protective characteristics of the chromated hybrid alloy coatings.
Acknowledgements
The authors express their gratitude to the Bilateral project between the Bulgarian (Institute of Physical Chemistry) and Chinese (Ningbo Institute of Materials Technology and Engineering) Academies of Sciences. The scientific support of Prof. DSc Petar Petrov (BAS – Institute of Polymers) is also gratefully acknowledged.
Literatur
[1] Kangas, P.: High Temperature Corrosion Problems in Refineries, Chemical Process Industries and Petrochemical Plants, High Temperature Corrosion, World Scientific, 2016, 87–98
[2] Isimjan, T.T.; Wang, T.; Rohani, S.: A novel method to prepare superhydrophobic, UV resistance and anti-corrosion steel surface, Chemical Engineering Journal, 210 (2012) 182–187
[3] Hacıibrahimoglu, M.Y.; Yavuz, A.: Electrochemical and morphological study of zn-mn alloys deposited from boric acid electrolyte onto the carbon steel for corrosion protection, International Journal of Research in Engineering and Technology, 5 (2016) 11, 68–74
[4] Brenner, A.: Electrodeposition of Alloys. Principles and Practice, Academic Press, New York, 1963
[5] Fashu, S.; Gu, C.D.; Zhang, J.L.; Zheng, H.; Wang, X.L.; Tu, J.P.: Electrodeposition, Morphology, Composition, and Corrosion Performance of Zn-Mn Coatings from a Deep Eutectic Solvent, Journal of Materials Engineering and Performance, 24 (2015) 1, 434–444
[6] Assaf, H.F.; Abou-Krisha, M.M.; Alduaij, O.K.; El-Seidy, A.M.A.; Eissa, A.A.: The Effect Manganese Concentration on the Corrosion Resistance and Physical Properties of Zn-Ni-Mn Alloy Films Produced by Electrodeposition, International Journal of Electrochemical Science, 10 (2015) 6273–6287
[7] Tafreshi, M.; Allahkaram, S.R.; Farhangi, H.: Comparative study on structure, corrosion properties and tribological behavior of pure Zn and different Zn-Ni alloy coatings, Materials Chemistry and Physics, 183 (2016), 263–272
[8] Azizi, F.; Kahoul, A.: Electrodeposition and corrosion behaviour of Zn/Co coating produced from a sulphate bath, Transactions of the Institute of Metal Finishing, 94 (2016), 1, 43–48.
[9] Karahan, I.H.: Effect of borax pentahydrate addition to acid bath on the microstructure and corrosion resistance of Zn-Co Coating, Acta Physica Polonica A, 128 (2015) 2, 432–435
[10] Ganesan, S.; Prabhu, G.; Popov, B.: Electrodeposition and characterization of Zn-Mn coatings for corrosion protection, Surface and Coatings Technology, 238 (2014) 143–151
[11] Winiarski, J.; Tylus, W.; Szczygiel, B.: EIS and XPS investigations on the corrosion mechanism of ternary Zn–Co–Mo alloy coatings in NaCl solution, Applied Surface Science, 364, 28 (2016) 455–466
[12] Tafreshi, M.; Allahkaram, S.R.; Farhangi, H.: Comparative study on structure, corrosion properties and tribological behavior of pure Zn and different Zn-Ni alloy coatings, Materials Chemistry and Physics, 183 (2016) 263–272
[13] Boshkov, N.; Vitkova, S.; Petrov, K.: Corrosion Products of Zn-Mn Coatings: Part I. Investigations Using Microprobe Analysis and X-Ray Diffraction, Metal Finishing, 99 (2001) 9, 56–60
[14] Boshkov, N.: Galvanic Zn-Mn alloys – electrodeposition, phase composition, corrosion behaviour and protective ability, Surface and Coatings Technology, 172 (2003) 2–3 , 217–226
[15] Boshkov, N.; Petrov, K.; Kovacheva, D.; Vitkova, S.; Nemska, S.: Influence of the alloying component on the protective ability of some zinc galvanic coatings, Electrochimica Acta, 51 (2005) 1, 77–84
[16] Boschkov, N.; Petrov, K.; Nikolova, S.; Vitkova, S.: Galvanische Zink-Mangan Legierungen – Elektrochemische Abscheidung und Strukturcharakteristika, Metalloberfläche, 52 (1998) 7, 514–519
[17] Tomachuk, C.R.; Elsner, C.I.; Di Sarli, A.R.; Ferraz, O.B.: Corrosion resistance of Cr (III) conversion treatments applied on electrogalvanised steel and subjected to chloride, Mathematics and Chemical Physics, 119 (2010), 19–29
[18] Tomachuk, C.R.; Di Sarli, A.R.; Elsner, C.I.: Behavior of electrogalvanized steel pre-treated with Cr (III)-based baths and exposed to 0,5 M Na2SO4 solution, Portugaliae Electrochimica. Acta, 30 (2012) 3, 145–162
[19] Cho, K.W.; Rao, V.S.; Kwon, H.S.: Microstructure and electrochemical characterization of trivalent chromium-based conversion coating on zinc, Electrochimica. Acta, 52 (2007), 4449–4456
[20] Zhang, X.; Sloof, W.G.; Hovestad, A.; Van Westing, E.P.M.; Terryn, H.; De Wit, J.H.W.: Characterization chromate conversion coatings on zinc using XPS and SKPFM, Surface and Coatings Technology, 197 (2005), 168–176
[21] Berger, R.; Bexell, U.; Grekh, T.M.; Hornstrom, S.E.: A comparative study of the corrosion protective properties of chromium and chromium free passivation methods, Surface and Coatings Technology, 202 (2007), 391–397
[22] Tomachuk, C.R.; Di Sarli, A.R.; Elsner, C.I.: Anti-corrosion performance of Cr (VI)-free passivating layers applied on electrogalvanised steel, Materials. Science and Applications, 1 (2010) 202–209
[23] Gabrielli, C.; Keddam, M.; Minoutlet-Laurent, F.; Ogle, K.; Perrot, H.: Investigation of zinc chromatation. I. Application of QCM-ICP coupling, Electrochimica Acta, 48 (2003), 965–976
[24] Bellezze, T.; Roventi, G.; Fratesi, R.: Electrochemical study on the corrosion resistance of Cr (III)-based conversion layers on zinc coatings, Surface and Coatings Technology, 155 (2002), 221–230
[25] Zhang, X.; Van den Bos, C.; Sloof, W.G.; Hovestad, A.; Terryn, H.; De Wit, J.H.W.: Comparison of the morphology and corrosion performance of Cr (VI)- and Cr (III)-based conversion coatings on zinc, Surface and Coatings Technology, 199 (2005), 92–104
[26] Boshkov, N.; Tsvetkova, N.; Petrov, P.; Koleva, D.; Petrov, K.; Avdeev, G. et al.: Corrosion behavior and protective ability of Zn and Zn-Co electrodeposits with embedded polymeric nanoparticles, Applied Surface Science, 254 (2008) 17, 5618–5625
[27] Koleva, D.; Boshkov, N.; Bachvarov, V.; Zhan, H.; de Wit, J.H.W.; van Breugel, K.: Application of PEO113-b-PS218 nano-aggregates for improved protective characteristics of composite zinc coatings in chloride containing environment, Surface and Coatings Technology, 204 (2010), 3760–3772
[28] Koleva, D.; Boshkov, N.; Raichevski, G.; Veleva, L.: Electrochemical corrosion behavior and surface morphology of electrodeposited zinc, zinc-cobalt and their composite coatings, Transactions of the Institute of Metal Finishing, 83 (2005) 4, 188–193
[29] Koleva, D.; Zhang, X.; Petrov, P.; Boshkov, N.; van Breugel, K.; de Wit, J.H.W. et al.: Zinc composite layers, incorporating polymeric nano-aggregates: surface analysis and electrochemical behavior, ECS Transactions, 11 (2008), 11, 27–35
[30] Koleva, D.; Taheri, P.; Tsvetkova, N.; Boshkov, N.; van Breugel, K.; de Wit, J.H.W. et al.: Corrosion performance of composite galvanic coatings with variable concentration of polymeric nano-aggregates and/or Cr (III) conversion layers, ECS Transactions, 33 (2011) 35, 85–92
[31] Boshkov, N.; Koleva, D.; Petrov, P.; Tsvetkova, N.: Engineered Metal Matrix Composites: Forming Methods, Material Properties and Industrial Applications, Chapter 10, Corrosion resistant nano-composite metallic coatings with embedded polymeric aggregates, NOVA Publishing House, (2013) 1, 261–282
[32] Petrov, P.; Bozukov, M.; Tsvetanov, C.: Innovative appro ach for stabilizing poly (ethylene oxide)-b-poly (propylene oxide)-b-poly (ethylene oxide) micelles by forming nano-sized networks in the micelle, Journal of Material Chemistry, 15, (2005), 1481–1486
[33] Guglielmi, N.: Kinetics of the deposition of inert particles from electrolytic baths, Journal of the Electrochemical Society, 119 (1972), 1009–1012
[34] Karlapper, A.M.J.; Foster, J.: A study of the mechanism of formation of electrodeposited composite coatings, Transactions of the Institute of Metal Finishing, 51, (1973) 1, 5287–5293
[35] Bercot, P.; Pena-Munoz, E.; Pagetti, J.: Electrolytic composite Ni-PTFE coatings: an adaptation of Guglielmi’s model for the phenomena of incorporation, Surface and Coatings Technology, 157 (2002), 282–289
[36] Roos, J.R.; Celis, J.-P.; Fransaer, J.; Buelens, C.: Mechanism of electrolytic composite plating: survey and trends, Transactions of the Institute of Metal Finishing, 69 (1991) 4, 133–139
[37] Stern, M.; Geary, A.L.: Electrochemical polarization I. A theoretical analysis of the shape of polarization curves, Journal of the Electrochemical Society, 104 (1957), 56–63
[38] Boshkov, N.; Petrov, K.; Vitkova, S.: Corrosion Products of Zn-Mn Coatings; Part III. Double-Protective Action of Manganese, Metal Finishing, 100 (2002) 6, 98–102
[39] Boshkov, N.; Nemska, S.; Vitkova, S.: Corrosion Products of Zn-Mn Coatings; Part II. Investigations Using X-Ray Photoelectron Spectroscopy, Metal Finishing, 100 (2002) 5, 14–20