Magnesium has a hexagonal close-packed (hcp) crystalline structure; it lacks ductility when worked at lower temperatures. Furthermore, in its pure polycrystalline form, it does not have sufficient strength for most structural applications. The metal is brittle and easily fractures along shear bands due to strain localization. Because of these reasons, pure magnesium must be alloyed with other elements to impart strength, weldability, formability, corrosion resistance, etc. In this article, types of magnesium alloys and their nomenclature system is discussed.
Assembly at chainsaw manufacturer Stihl. Using magnesium die-casting, the company in Weinsheim in Rhineland-Palatinate manufactures magnesium alloy components that ensure the lightness and stability of the company's products
Aluminium, manganese and zinc are the main alloying elements in magnesium alloys. The following is the significance of addition of various alloying elements [1-4]:
- Aluminium is the most common alloying element. It improves strength, hardness, and corrosion resistance but reduces ductility. Addition of 5-6 % yields the optimum combination of strength and ductility. Further addition makes it easier to cast, but more difficult to extrude due to increased hardness
- Zinc increases strength, and fluidity in casting, but reduces ductility
- Manganese increases the corrosion resistance of Mg-Al-Zn alloys by forming intermetallic compounds with iron group metals, some of which separate out during melting and are removed
- Rare earth metals provide precipitation strengthening and can improve both strength and ductility through randomization of crystallographic texture. Rare earths can also improve resistance to high-temperature creep
- Zirconium is added as a strong grain refiner to alloys containing zinc and rare earth metal.
- Iron, copper and nickel reduce the corrosion resistance of magnesium alloys. iron must be kept below 50 ppm
- Lithium is found to enhance formability, reduces density and can improve ductility by creating a cubic structure at over 11 % additions. Addition of lithium significantly increases cost and reduces corrosion resistance
- Silicon increases fluidity and creep strength
- Rare earths (e.g., Th, Y), Ca and Sr have shown to increase creep resistance
- Small amount of calcium significantly reduces the flammability
- Tin increases ductility, and improves castability
- Beryllium decreases surface oxidation during casting and welding.
The aluminium-containing magnesium alloys are usually used for casting and the zirconium-containing ones for forgings. Mg-Al-Zn group alloys are used for room temperature applications, while Mg-Zn-Zr group alloys (without aluminium) are used at elevated temperatures [5].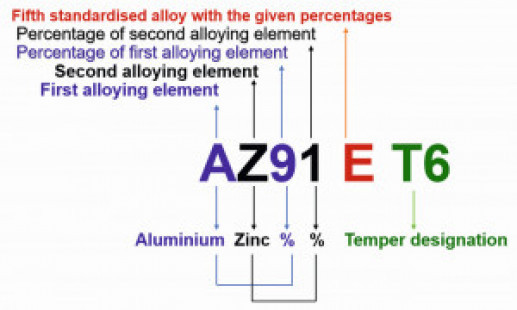 Fig. 1: ASTM nomenclature of magnesium alloys
Fig. 1: ASTM nomenclature of magnesium alloys
Alloys Nomenclature
A standard designation system for magnesium alloys was adopted in 1948 as per ASTM B 275. The ASTM designations are based upon the chemical composition related information, and have four parts symbolized as letter-letter, number-number, letter, and temper code. The following letters designate various alloying elements: A, aluminium; B, bismuth; C, copper; D, cadmium; E, rare earths; F, iron; H, thorium; K, zirconium; L, lithium, M, manganese; N, nickel; Q, silver; R, chromium, S, silicon; T, tin; W, yttrium; and Z, zinc. For instance, the designation of magnesium alloy AZ91E-T6 illustrated in Figure 1, can be explained as follows [2,6]:
- The first part letter-letter (AZ) represents the two main alloying elements (aluminium, zinc), specified in the greatest amount, arranged in decreasing percentages, or alphabetically if of equal percentage
- Second part number-number (91) signifies the percentage amount of the main alloying elements (9% and 1%, respectively) rounded off to whole numbers
- Third part letter (E) differentiates alloys having the same amounts of the main alloying elements (fifth standardised alloy with the above percentages). If there is letter X, it denotes the experimental alloy
- Fourth part (T6) designates the condition of the alloy i.e., temper (solution heat-treated and artificially aged)
- The composition of some typical magnesium alloys is shown in Table 1 [1].
Alloy
Use
% Composition by weight; remarks
AM20
D
Al:2.0, Mn: 0.40 min., Zn:0.2 max., Fe:0.005 max., Ni:0.002 max.;
high ductility and impact strengthAM50
D
Al:5.0, Mn: 0.26 min., Zn:0.2 max., Fe:0.005 max., Ni:0.002 max.;
high-energy absorption and elongationAM60B
D
Al:6.0, Mn: 0.24 min., Zn:0.2 max., Fe:0.005 max., Ni:0.002 max.;
excellent ductility, superior energy absorbing properties, and good strengthAE42
D
Al:4.0, RE:2.5 (Ce-rich), Zn:0.2 max., Mn: 0.1 min., Fe:0.005 max.,
Ni: 0.002 max.; good strength and creep resistance up to 150 °CAS21
D
Al:2.0, Si:1.0, Zn:0.2, Mn: 0.2, Fe:0.005 max., Ni:0.002 max.;
good strength and its creep resistance is up to 150 °CAS41B
D
Al:4.0, Si:1.0, Mn: 0.35, Zn:0.2 max., Fe:0.005 max., Ni:0.002 max.;
good tensile strength and elongationAZ31B
W
Al:3.0, Zn:1.0, Mn: 0.20 min.; good room-temperature strength, ductility,
corrosion resistance and weldabilityAZ63
P
Al:6.0, Zn:3.0, Mn: 0.15 min.; good strength at room temperature
AZ80
W
Al:8.0, Zn:0.2, Mn: 0.12 min.; high strength alloy
AZ91D
D, S, P
Al:9.0, Zn:0.7, Mn: 0.15 min.; excellent combination of mechanical properties,
corrosion resistance, and castabilityEQ21
S
RE: 2.1 (Nd-rich), Ag: 1.5, Zr:0.5; high strength casting alloy
EZ33
S
RE:3.3 (Ce-rich), Zn:2.7, Zr:0.6; military pressure-tight sand and
permanent-mould castingsHK31
S
Th: 2.5, Zr:0.4 min., Zn: ≤0.3; high creep resistance to 350 °C, weldable
QE22
S
Ag:2.5, RE:2.1 (Nd-rich), Zr:0.7; high strength casting alloy
WE43
S, W
Y:4.0, RE:3.0 (Nd-rich), Zr:0.5; high temperature creep resistance
WE54
S
Y:5.3, RE:3.5 (Nd-rich), Zr:0.5; high temperature creep resistance
ZE41
S
Zn:4.2, RE:1.2 (Ce-rich), Zr:0.7; ideal for high integrity castings
ZK30A
W
Zn:3.0, Zr:0.6; high strength alloy
ZK60A
W
Zn:6.0, Zr:0.4; good formability
ZM21
W
Zn:2.0, Mn:1.0; good damping capacity, medium strength and good forgeability
LA141
W
LI:14.0, Al: 1.0; ultra-light, lightest metal structure material, d~1.35 g/cm3
D= Diecast, S= Sandcast. P= Permanent Mould, W= Wrought Product
Tab.1: Magnesium alloys. Nominal compositions, wt.%
Temper Designation
The tempers defined in ASTM B 296 and BS-EN 1559-5 are used for all magnesium alloy products except ingots and are based on the sequence of basic treatments used to produce the various tempers.
Though the alloy nomenclature system for magnesium alloys varies in different regions across the globe, the temper designation system is quite similar to that of aluminium alloys, BS-EN-515 2017. The temper designations consist of letters. Subdivisions of the basic tempers are indicated by digit(s) following the letter.
The basic temper designations and the subdivisions of magnesium alloys are briefly explained in Table 2 [1].
|
F |
As fabricated. |
|
O |
Soft annealed, recrystallized (wrought products only). |
|
H |
Strain-hardened (wrought products only) |
|
H1 |
Strain-hardened only to obtain the desired mechanical properties. |
|
H2 |
Strain-hardened more than the desired and then partially annealed to lower the strength to as desired. |
|
H3 |
Strain-hardened and then stabilized by a low temperature heating to slightly lower their strength and increase ductility. |
|
Subdivisions of the H1, H2 and H3 Tempers The second digit following H1, H2 and H3 designation indicates the final degree of strain-hardening. |
|
|
W |
Solution heat-treated, unstable temper. Applicable to alloys which spontaneously age at room temperature after solution heat treatment. The period of natural aging e.g., W1/2 hr. should be indicated. |
|
T |
Heat treated to produce stable tempers other than F, O, or H. T is followed numerals 1 through 10 to |
|
T1 |
Cooled from an elevated temperature shaping process and naturally aged at room temperature. |
|
T2 |
Annealed to improve ductility and increase stability of castings (cast products only). |
|
T3 |
Solution heat-treated and then cold worked to improve strength. |
|
T4 |
Solution heat-treated and naturally aged. |
|
T5 |
Cooled from an elevated-temperature shaping process such as casting or extrusion, and then artificially aged to improve mechanical properties or dimensional stability or both. |
|
T6 |
Solution heat-treated and artificially aged. |
|
T7 |
Solution heat-treated and stabilized to control special characteristics. |
|
T8 |
Solution heat-treated, cold worked and then artificially aged. |
|
T9 |
Solution heat-treated, artificially aged and cold worked to improve strength. |
|
T10 |
Cooled from thermomechanical treatment, artificially aged, and cold worked. |
|
Additional digits may be added to T1 to T10 designation to indicate a variation in treatment that significantly |
|
Tab. 2: Temper designations of magnesium alloys
ASTM B296-20 (05-01-2020): Standard Practice for Temper Designations of Magnesium Alloys, Cast and Wrought standard by ASTM International
BS-EN 1559-5 (05/01/2017): Founding - Technical conditions of delivery - Part 5: Additional requirements for magnesium alloy castings. This part does not apply to ingots, bars, billets (or other shapes) for further reprocessing, such as re-melting or extrusion.
BS-EN 515:2017 (31-03-2017):Aluminium and aluminium alloys. Wrought products. Temper designations. Published by British Standards Institution.
Magnesium alloys with very low density and good formability have been developed by adding lithium to magnesium. For example, LA141A, which contains 14 % Li, has a density of 1.35 g/cm3, 22 % less than that of pure magnesium [7]. Lithium addition to magnesium has an important effect on the lattice structure. Mg-Li alloys exhibit two phase structures of Mg-rich α (hexagonal close packed, hcp) and Li-rich β (body-centered cubic, bcc) phases at room temperature when 5-11 % Li is added to magnesium. Further additions of Li (over 11 %) can transform the hcp α-Mg solid solution into highly workable, body-centered cubic alloys [8]. The brittle-to-ductile transition coupled with lithium’s lower density of 0.58 g/cm3 presents substantial benefits of using Mg-Li based alloys for weight saving purposes [9-11].
Magnesium-lithium alloys are referred to as superlight materials, and are the lightest metallic engineering materials [12-14]. However, due to inclusion of highly reactive lithium metal, these alloys have found very limited use in missile and aerospace applications because of its ultra-low density and excellent formability at room temperature or slightly elevated temperatures [15].
REFERENCES:
[1] Westengen, H.; Rashed, H.: Magnesium Alloys: Alloy and Temper Designation System, Reference Module in Materials Science and Materials Engineering, Elsevier, (2016), 1-3. doi: 10.1016/B978-0-12-803581-8.02567-4
[2] Sandlobes, S.; Friak, M.; Korte-Kerzel, S.; Pei, Z.; Neugebauer, J.; Raabe, D.: A rare-earth free magnesium alloy with improved intrinsic ductility, Sci. Rep., 7, no. 1, (2017), 10458. doi: 10.1038/s41598-017-10384-0
[3] Powell, B.R.; Krajewski, P.E.; Luo, A.A.: Magnesium alloys for lightweight powertrains and automotive structures (Chapter 4), Materials, Design and Manufacturing for Lightweight Vehicles, P.K. Mallick (Editor), Second Edition, Woodhead Publishing, (2021), 125-186. doi: 10.1016/B978-0-12-818712-8.00004-5
[4] Ghali, E.: Corrosion Resistance of Aluminum and Magnesium Alloys: Understanding, Performance, and Testing, John Wiley & Sons, (2010), 1-752
[5] Ghassemieh: Materials in Automotive Application, State of the Art and Prospects (Chapter 20), New Trends and Developments in Automotive Industry, IntechOpen, (2011), 365-394. doi: 10.5772/13286
[6] Moosbrugger, C. (Editor): Introduction to Magnesium Alloys (Chapter 1), Engineering Properties of Magnesium Alloys, ASM International, Ohio, (2017), 1-10
[7] Luo, A.A.; Sachdev, A.K.: 12 - Applications of Magnesium Alloys in Automotive Engineering, Advances in Wrought Magnesium Alloys, Woodhead Publishing, (2012), 393-426. doi: 10.1533/9780857093844.3.393
[8] Jackson, J.H.; Frost, P.D.; Loonam, A.C.; Eastwood, L.W.; Lorig, C.H.: Magnesium-lithium base alloys - preparation, fabrication, and general characteristics, J. Met. Mater. Miner., 1 no. 2, (1949), 149-168. doi: 10.1007/bf03398089
[9] Yang, C.W.: Tensile Mechanical Properties and Failure Behaviours of Friction Stir Processing (FSP) modified Mg-Li-Zn and dual-phase Mg-Li-Al-Zn alloys, InTech, 10, (2013), 303-336. doi: 10.5772/54313
[10] Shin, I.; Carter, E.A.: First-principles simulations of plasticity in body-centered-cubic magnesium-lithium alloys, Acta Mater., 64, (2014), 198-207. doi: 10.1016/j.actamat.2013.10.030
[11] Al-Samman, T.: Comparative study of the deformation behavior of hexagonal magnesium-lithium alloys and a conventional magnesium AZ31 alloy, Acta Mater., 57, no. 7, (2009), 2229-2242. doi: 10.1016/j.actamat.2009.01.031
[12] Chakravorty, C.R.: Development of ultra light magnesium-lithium alloys, Bull. Mater. Sci., 17, no. 6, (1994), 733-745. doi: 10.1007/bf02757554
[13] Singh, S.K.; Chakravorty C.R.: Advanced magnesium based alloys containing lithium - Recent developments, J. Inst. Engineers (India), Part MM, 79, no. 1, (1998), 11-15
[14] Wu, R.; Zhang, Z. W.: Recent progress in magnesium-lithium alloys, Int. Mater. Rev., 60, no. 2, (2015), 65-100. doi: 10.1179/1743280414y.0000000044
[15] Muga, C.O.; Zhang, Z. W.: Strengthening mechanisms of magnesium-lithium based alloys and composites, Adv. Mater. Sci. Eng., (2016), 1078187, 1-11. doi: 10.1155/2016/1078187


